Antibacterial technology development support
Development of applied antibacterial products using earthplusTM
We frequently partner with firms exploring the development of antibacterial products.
The antibacterial properties offered by earthplusTM can be combined with materials used in various industries to create new added value in product development, enhancing the value proposition of your business and offering greater safety and peace of mind to your users.
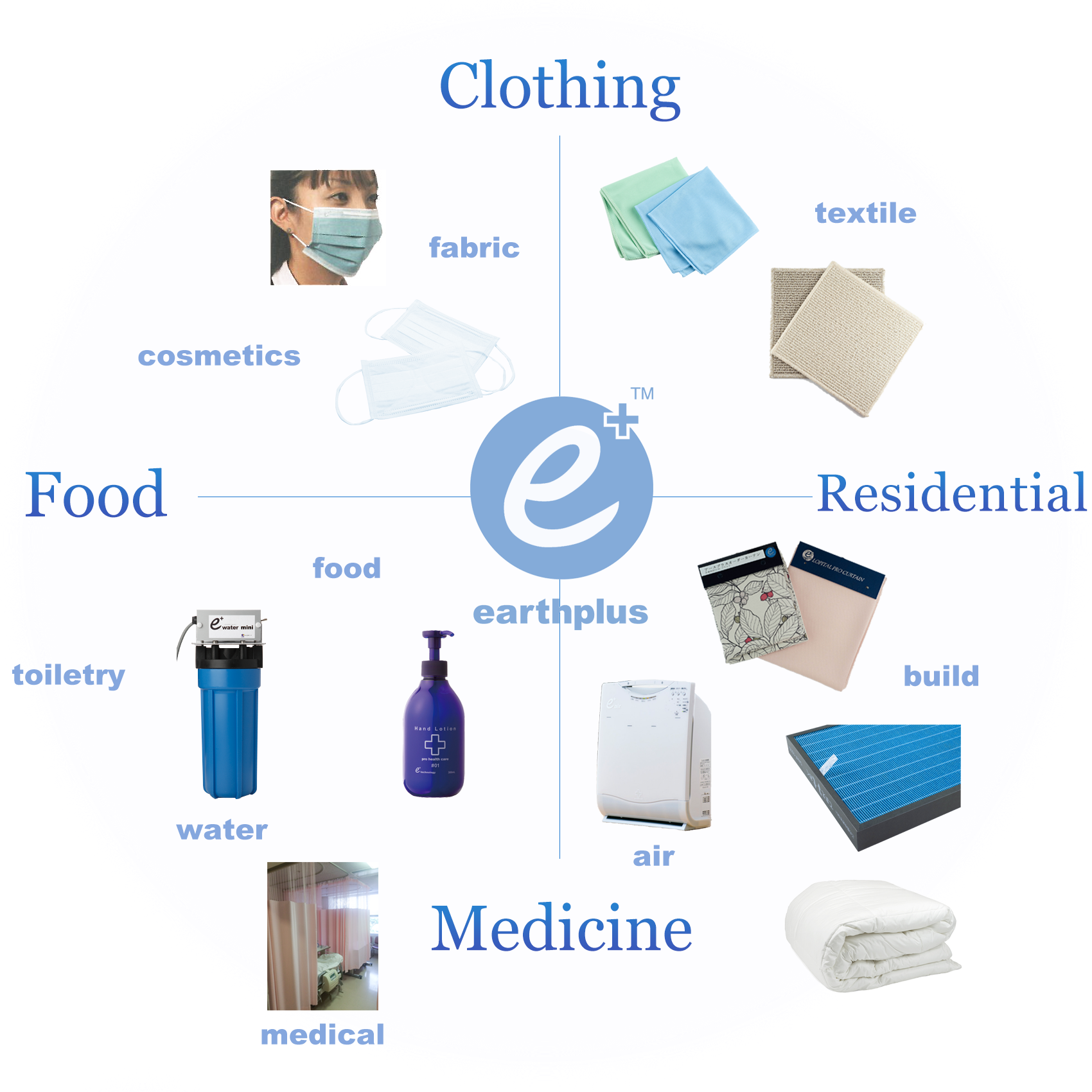
earthplusTM applicable fields

- Curtains
- Masks
- Linen/fabric
- Air purifier filters
- Residential equipment/fixtures
- Food packaging
- Antibacterial curtains
- Antibacterial masks
- Antibacterial linen/fabric
- Antibacterial air purifiers
- Antibacterial wallpaper
- Antibacterial packaging
earthplusTM applications


- Curtains
- Uniforms
- Sports
- Socks
- Underwear
- Pet goods

- Foundation
- Concealer
- Skincare bases


- Functional packaging materials
- Antiseptics/preservatives

- Treating plated surfaces to resist degradation when washed


- Residential equipment/fixtures
- Tiles
- Paints/coatings
- Ceiling materials
- Interior materials
- Kitchen equipment

- Air conditioner filters
- Humidifiers
- Dehumidifiers
- Air conditioning and heating


- Lab coats
- Hospital gowns
- Bedsheets
- Medical equipment

- Carpeting
- Bedding
- Linens

- Antiseptic/eliminating bacteria
- Hygienic/sanitary goods
Development of antibacterial products using earthplusTM



earthplusTM mechanisms
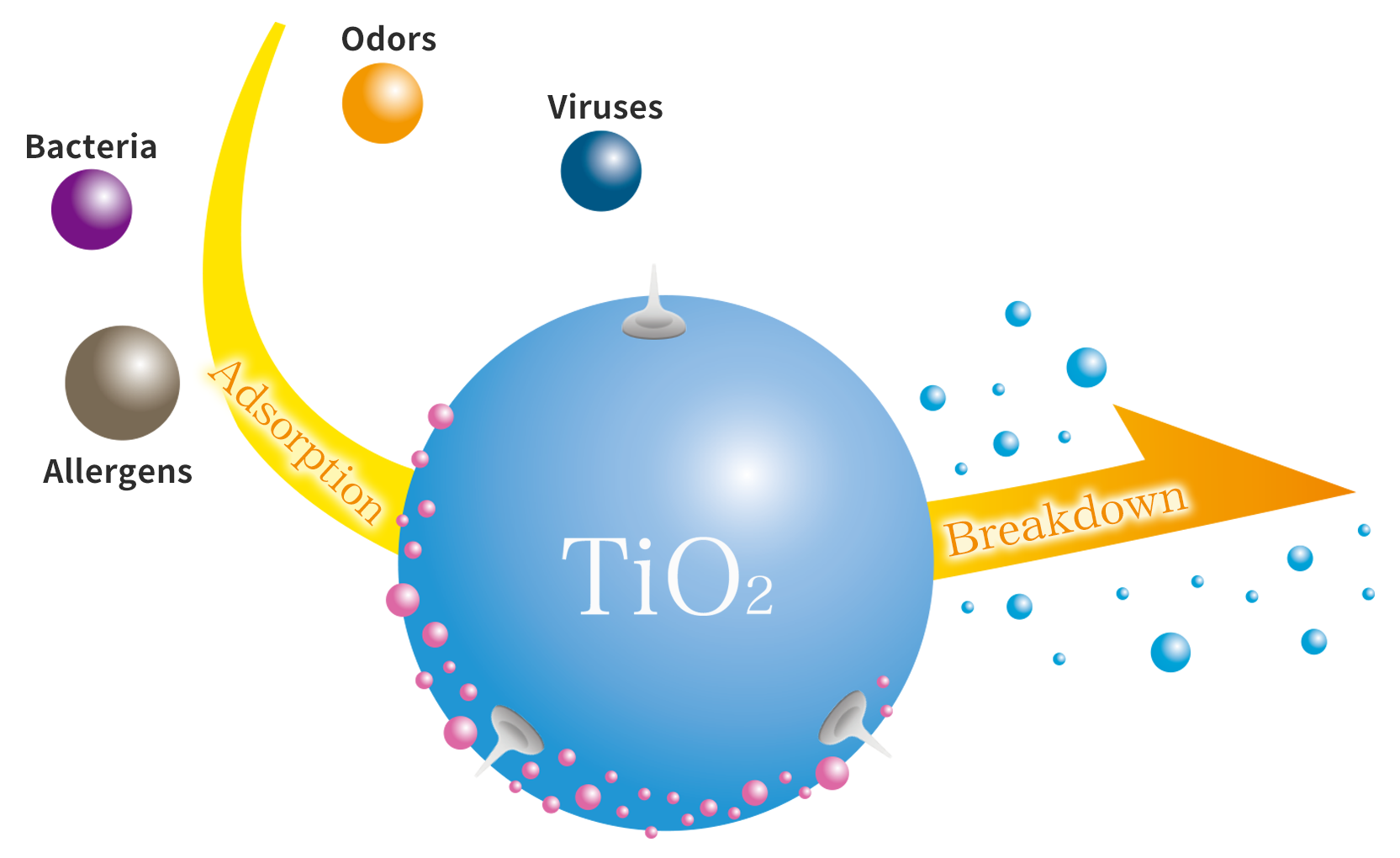
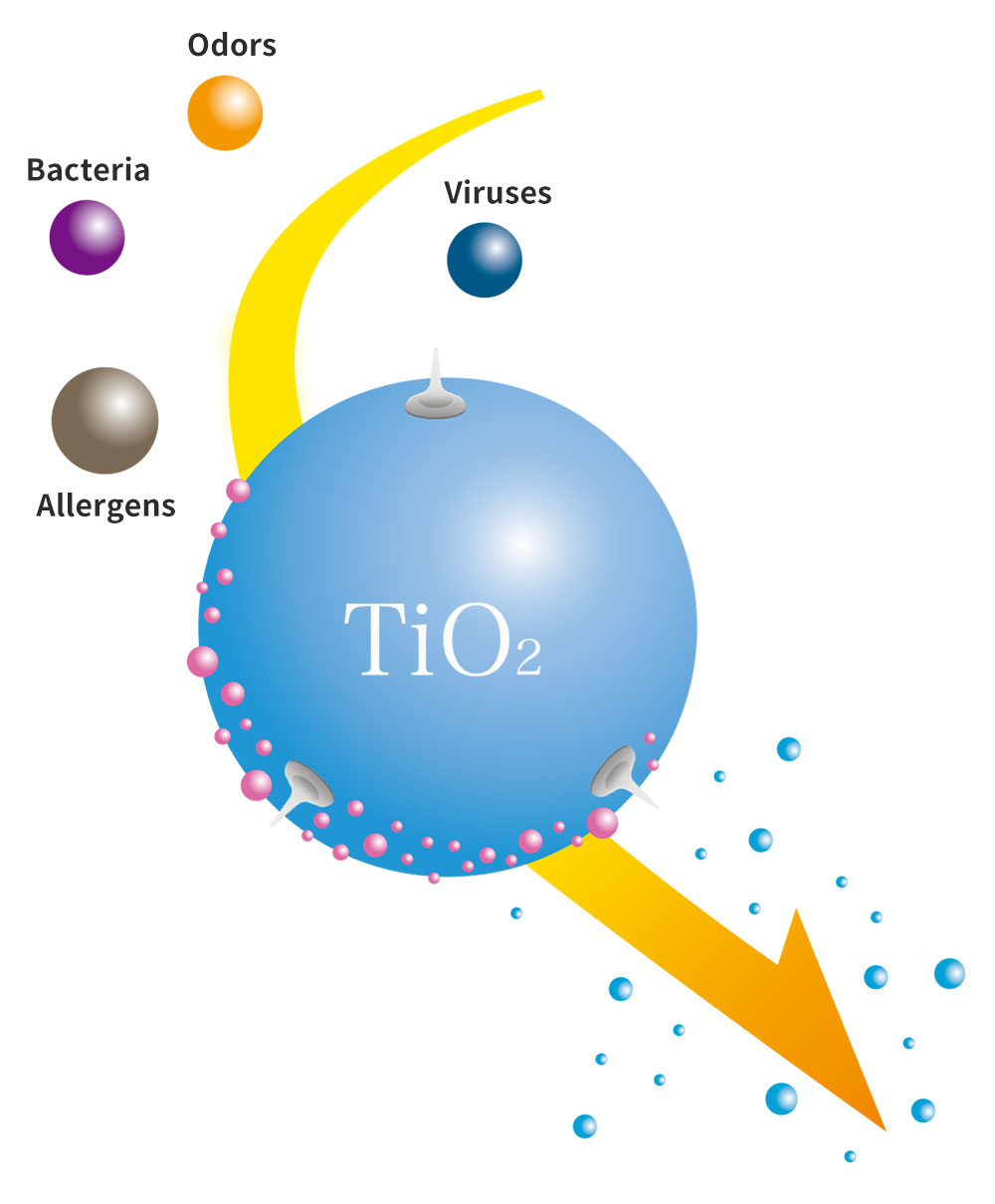
-
TiO2
Titanium oxide

This material decomposes proteins such as bacteria, viruses and odors into harmless substances.
-
HAp
Hydroxyapatite

This material absorbs the bacteria and viruses, and will not release them.
-
Ag
Electrode
The silver is the electrode that speeds up the titanium oxide during the decomposition process.
Photograph (taken with an electron microscope) of earthplusTM breaking down Staphylococcus aureus



Microbial attributes with which we have past experience
- MRSA
- Staphylococcus aureus
- ESBL-producing E.coli
- MBL-producing Pseudomonas
- Tricophyton fungus
- O-157
- Candida
- Legionella
- Klebsiella pneumoniae
- Influenza virus/li>
- Feline calicivirus
- Cedar allergen
- Tick allergen
We were recognized by a German institute for our product’s outstanding antibacterial properties.
Its efficacy was demonstrated by this institute in Germany.
We cleared the international ISO20743 standard for textile products antibacterial evaluation.
Antibacterial properties against Staphylococcus aureus
| Substrate | After inoculation | 18 hours later | Decrease rate |
|---|---|---|---|
| Window curtains | 232,000 | 815 | 99.6%+ |
| Lace curtains | 232,000 | 19 | 99.9%+ |
| Bed curtains | 232,000 | 19 | 99.9%+ |
| Untreated | 232,000 | 2,740,000 | – |
Antibacterial properties against Klebsiella pneumoniae
| Substrate | After inoculation | 18 hours later | Decrease rate |
|---|---|---|---|
| Window curtains | 50,200 | 556 | 98.8%+ |
| Lace curtains | 50,200 | 1,650 | 96.7%+ |
| Bed curtains | 50,200 | 19 | 99.9%+ |
| Untreated | 50,200 | 24,000,000 | – |
Antibacterial to MRSA
| Substrate | After inoculation | 18 hours later | Decrease rate |
|---|---|---|---|
| Window curtains | 198,000 | 3,270 | 98.3%+ |
| Lace curtains | 198,000 | 19 | 99.9%+ |
| Bed curtains | 198,000 | 140 | 99.9%+ |
| Untreated | 198,000 | 5,180,000 | – |

Certification tag issued by the Hohenstein Institute
*Performed against SEK mark red label, a certification standard of bacterial treatment for specific use at medical institutions.
- Testing sites
- Hohenstein Institute, Germany
- Test modalities
- DIN EN ISO 20743A,Z:2007-10
- Test bacteria
-
Staphylococcus aureus ATCC6538 Staphylococcus aureus ATCC 6538
Klebsiella pneumoniae ATCC4352
MRSA Staphylococcus aureus(MRSA) ATCC33592
Three reasons why Shinshu Ceramics creates safe and secure solutions
Over 20 years of proven experience in antibacterial and odor eliminating solutions at medical institutions, which call for advanced technology
- We have developed and provided antibacterial and odor eliminating products specifically for medical organs
- We have a proven track record of technical excellence and performance clearing stringent standards for use in medical institutions
- We have over twenty years of results in developing antibacterial and odor eliminating materials
Quality control system
Viral and bacterial testing chambers
We maintain a variety of bacterial and virus inspection and quality control equipment that are used to pursue ongoing quality improvements.
This system allows any product to offer the full earthplusTM quality and functionality and do so at a high standard of safety and security.

We support your research team or new product development workflow with our experience and expertise.
Our stance to development is that we are uniquely poised to solve a range of challenges through our demonstrated knowledge and experience. We will boldly attempt new products with you and deliver them to success.
Let us work together to develop safe and secure solutions.

Product development flow
- Meeting
- Proposal
- Estimate and contract
- Research and development
- Commodification
Click here for enquiries
81-264-55-1221
FAX. 81-264-55-1181
Enquiries by e-mail