Antibacterial effects and safety data
Table of contents
Evaluation data
- Antiviral test
- Antibacterial tests
- Deodorizing/odor resistance tests
- Determination of antibacterial activity of textile products (ISO20743)
- Measurement of antibacterial activity on plastics and other non-porous surfaces (ISO22196)
- Illuminance and antibacterial performance
- Tick allergen tests
- Cedar pollen allergen test results
Evaluation data
Antiviral test
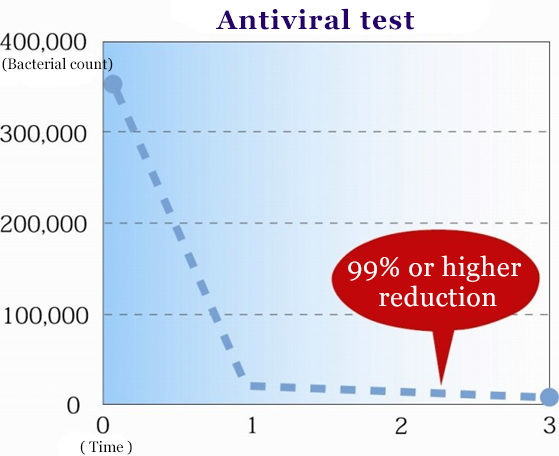
- Evaluative organs
- Biomedical Science Association (BMSA), an NPO
- Test modalities
- Measuring rate of infection using MDCK (canine liver cell) plaque assays against A/California 07/09 (H1N1Pdm)
Antibacterial tests
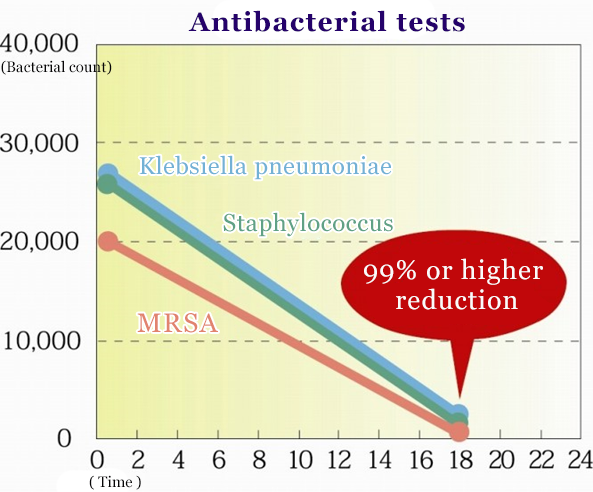
- Evaluative organs
- Boken Quality Evaluation Institute
- Test modalities
- JIS L 1902
- Test bacteria
- Staphylococcus aureus, Klebsiella pneumoniae, MRSA
Deodorizing/odor resistance tests
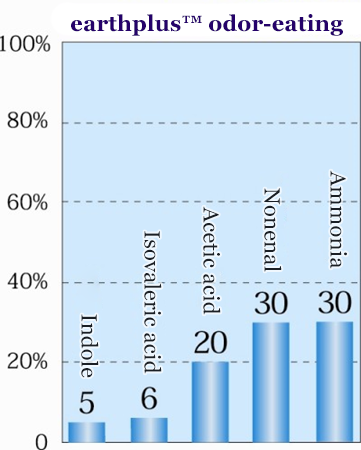
Deodorizing effect is from 2 hours after start of test
- Deodorizing performance test modality
- Japan Textile Evaluation Technology Council deodorant treated textile fiber approval standards analysis manual
Determination of antibacterial activity of textile products (ISO20743)
Antibacterial performance was confirmed after 100 washes.
Material was washed using test methodology (high temperature accelerated washing) per the “specific use” defined for antibacterial treated textiles.
(Test performed using JAFET-compliant blended detergent)
Staphylococcus aureus (ATCC6538)
| Substrate | After inoculation | 18 hours later | Decrease rate |
|---|---|---|---|
| earthplusTM treated fabric | 239,000 | <20 | 99.9%+ |
| Untreated | 239,000 | 1,510,000 | – |
Pneumobacillus (ATCC4352)
| Substrate | After inoculation | 18 hours later | Decrease rate |
|---|---|---|---|
| earthplusTM treated fabric | 10,500 | <20 | 99.9%+ |
| Untreated | 10,500 | 1,750,000 | – |
MRSA (ATCC33592)
| Substrate | After inoculation | 18 hours later | Decrease rate |
|---|---|---|---|
| earthplusTM treated fabric | 368,000 | <20 | 99.9%+ |
| Untreated | 368,000 | 3,760,000 | – |

Certification tag issued by the Hohenstein Institute
*Performed against SEK mark red label, a certification standard of bacterial treatment for specific use at medical institutions.
- Results
- Antibacterial performance was observed in the fabric after 100 washes.
- Evaluative organs
- Hohenstein Institute, Germany
- Test modalities
- DIN EN ISO 20743A,Z:2007-10
- Test bacteria
-
Staphylococcus aureus ATCC6538
Klebsiella pneumoniae ATCC4352
MRSA Staphylococcus aureus(MRSA) ATCC33592
Measurement of antibacterial activity on plastics and other non-porous surfaces (ISO22196)
MRSA (ATCC33592)
| Substrate | After inoculation | 18 hours later | Decrease rate |
|---|---|---|---|
| earthplusTM melting type | 202,000 | <20 | 99.9%+ |
| earthplusTM paint model | 95,300 | <20 | 99.9%+ |
E. coli (ATCC8739)
| Substrate | After inoculation | 18 hours later | Decrease rate |
|---|---|---|---|
| earthplusTM melting type | 85,600 | <20 | 99.9%+ |
| earthplusTM paint model | 264,000 | 1,622 | 99.3%+ |

Certification tag issued by the Hohenstein Institute
- Evaluative organs
- Hohenstein Institute, Germany
- Test modalities
- ISO 22196:2007-10
- Test bacteria
-
MRSA Staphylococcus aureus(MRSA) ATCC33592
E.coli ATCC8739
Illuminance and antibacterial performance
Differences in antibacterial properties were confirmed in terms of illuminance.
A change was confirmed (logarithmic value) in the number of live bacteria on the earthplusTM treated fabric and untreated fabric.
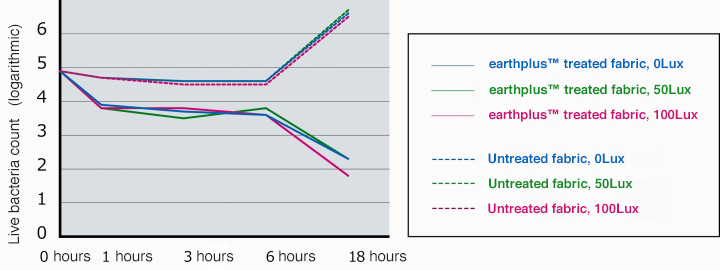
- Results
- No noticeable difference in antibacterial properties was observed in terms of light intensity. Antibacterial performance was observed at all light intensity levels.
- Test specimen
- earthplusTM treated fabric (paint model)
- Test modalities
- JIS L 1902
- Test bacteria
- Staphylococcus aureus (ATCC6538)
- Illuminance
- DIGITAL LUX METER MODEL:LX-1010B
Tick allergen tests
- Evaluative organs
- ITEA Co., Ltd. Tokyo Environmental Allergy Research Institute
- Test name
- Tick allergen (Der f 1) reaction test
- Test objective
- Measuring concentration of tick allergens over time on an earthplusTM treated nonwoven fabric
- Specimen
- earthplusTM treated nonwoven fabric
- Control
- Untreated
- Sample size
- 4×5[cm]
- Concentration
- 100[ng/ml]
- Reaction conditions
-
Temperature: 4 [Celsius]
Reaction time: 0/6/24 [hours]
- Amount added
- 1000 [μl] per sample
- Allergen solution
-
Tick allergen (Der f 1)
Allergen solution/crude tick extract
(Produced by ITEA; solution concentration: Der f 1:100ng/ml)
- Number of experimental samples
- n = 1 x 3 for each (carried out independently on each date)
- Measurement samples
- Allergen solution reacting to the sample
- Methodology
- A tick allergen solution (includes tick allergens) was applied to the sample and left under reaction conditions. The control was made only with allergens added to an untreated sample. After the given time elapsed, the sample was recovered, and the concentration in the allergen solution was measured.
- Allergen measurement
-
Enzyme immunoassay (Sandwich ELISA)
Primary solid phase antibodies were applied to each well of a 96-well microplate and supplemented with allergens. Next, a pre-labeled secondary antibody was reacted, followed by enzyme and substrate reactions. The absorbance of each colored well was measured and antigen amount of the specimen was measured against a standard curve.
Tick allergen (Der f 1) measurement results
(ng/ml)
| 0h | 6h | 24h | ||
|---|---|---|---|---|
| earthplusTM treated nonwoven fabric | ① | 58.31 | 11.29 | 6.123 |
| ② | 101.53 | 20.15 | 5.3 | |
| ③ | 90.86 | 0.41 | 0.10 | |
| Average | 83.57 | 10.62 | 3.84 | |
| Standard deviation | 22.51 | 9.89 | 3.27 | |
| Untreated | ① | 58.31 | 72.74 | 59.1 |
| ② | 101.53 | 118.08 | 115.74 | |
| ③ | 90.86 | 85.94 | 90.81 | |
| Average | 83.57 | 92.25 | 88.55 | |
| Standard deviation | 22.51 | 23.32 | 28.39 | |
Change in tick allergen concentration over time (average)
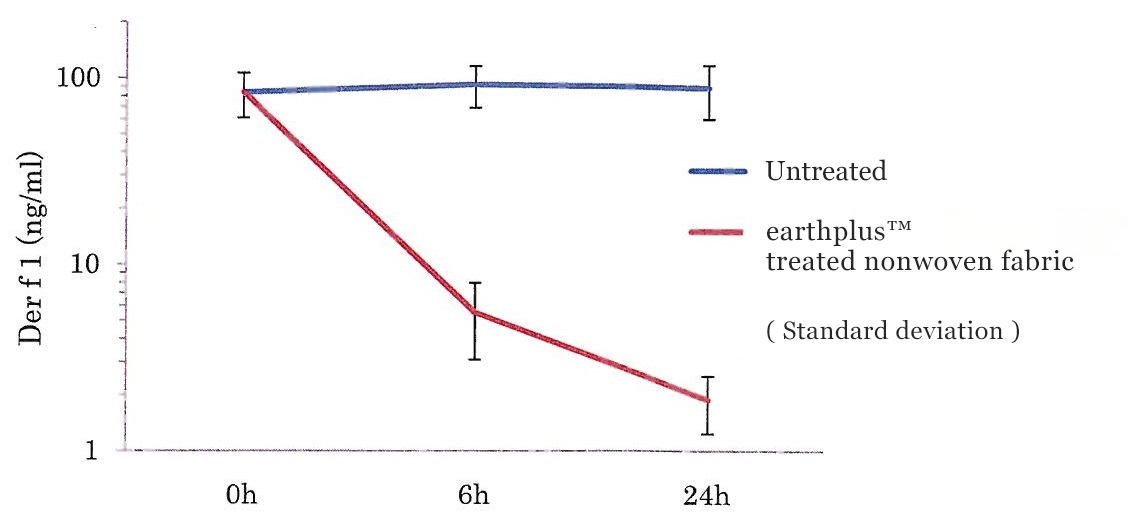
Notes
One reason for the decrease in allergens seen in the untreated sample was the effects of nonspecific adsorption and allergen decrease in components of the material.
Cedar pollen allergen test results
- Evaluative organs
- ITEA Co., Ltd. Tokyo Environmental Allergy Research Institute
- Test name
- Evaluating cedar pollen allergen reaction in earthplusTM treated nonwoven fabric
- Test objective
- Evaluating decrease in cedar pollen allergen concentration in earthplusTM treated nonwoven fabric
- Specimen
- earthplusTM treated nonwoven fabric
- Control
- Untreated
- Sample size
- 2×5[cm]
- Reaction conditions
-
Temperature: 4 [Celsius]
Other parameters: left overnight under fluorescent light in refrigerator
- Amount added
- 500[μl] per cloth sample
- Allergens measured
- Cedar pollen allergen (Cry j 1)
- Allergen solution
-
Crude allergen solution (produced by ITEA) dissolved in acetate buffer (Tween 20)
(Allergen concentration set at 100ng/ml)
- Number of experimental samples
- n = 3 for each
- Measurement samples
- Sample of recovered allergen solution reacted to the specimen
- Methodology
- A buffer containing allergen solution was added to the sample and concentration of allergen in the sample and solution post-contact was measured
- Allergen measurement
-
Enzyme immunoassay (Sandwich ELISA)
Primary solid phase antibodies were applied to each well of a 96-well microplate and supplemented with allergens. Next, a pre-labeled secondary antibody was reacted, followed by enzyme and substrate reactions. The absorbance of each colored well was measured and antigen amount of the specimen was measured against a standard curve.
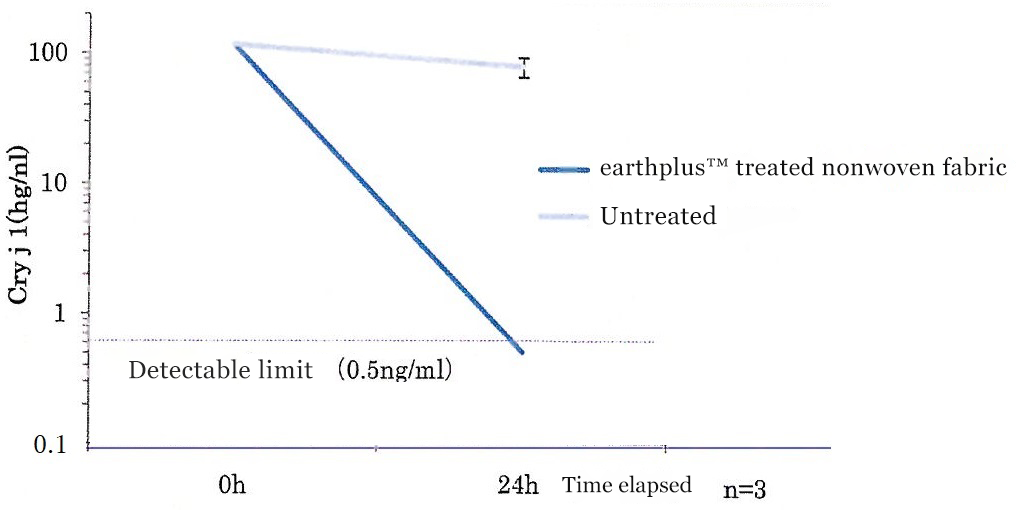
Cedar pollen allergen concentration
(ng/ml)
| 0h | 24h | |||
|---|---|---|---|---|
| Measured value | Average value | Standard deviation | ||
| earthplusTM treated nonwoven fabric | 116.14 | <0.50 | <0.50 | 0 |
| <0.50 | ||||
| <0.50 | ||||
| Untreated | 86.09 | 77.44 | 12.93 | |
| 67.80 | ||||
| 78.42 | ||||
Decrease in concentration of cedar pollen allergen
Safety data
Skin sensitivity
Skin sensitization test (adjuvant and patch test) using earthplusTM on guinea pigs
- Test number
- B020880
- Test outsourcee
- Shinshu Ceramics Co., Ltd.
- Test date
- 01/20/03
- Test performer
- Seiji Otake, Mitsubishi Safety Research Laboratories Toxicity Research Lab
Observations and conclusions
An adjuvant and patch test using earthplusTM was performed for this evaluation.
After stimulating the skin, both the animals in the group were administered the sample and the control group showed no reaction on the site of dosage. We believe this indicates that the test material does not cause skin reactivity under these test conditions.
Given that, compared to a positive control group, obvious skin reactions were found on DNCB-administered areas in all cases, the experimental procedures are found to be valid.
Per these findings, earthplusTM was found to not show skin reactivity in guinea pigs when administered under these test conditions.
Cytotoxicity tests
Cytotoxicity test using earthplusTM extract (colony-forming assay)>
- Test number
- B10757
- Test outsourcee
- Shinshu Ceramics Co., Ltd.
- Test date
- 01/20/03
- Test performer
- Seiji Otake, Mitsubishi Safety Research Laboratories Toxicity Research Lab
Observations and conclusions
An evaluation of earthplusTM cytotoxicity was performed using V79 cells in a colony-forming assay.
The colony-forming assay test found that rate of colony formation in the plate treated with the specimen extract decreased commensurate to the concentration of extract, suggesting cytotoxicity. IC50 of the specimen extract was 53.%% (95% confidence limit: 47.3-63.0%), a lower value compared to IC50 of standard material B (69.7%), but higher than IC50 of standard material A (2.3%). Therefore, we believe the cytotoxicity of the specimen is stronger than the standard material B, but lower than the standard material A.
The IC50 value of the extract from standard materials A and B was respectively <7% and <70%, showing no significant difference in number of colonies formed between the plate treated with negative material extract and the negative control plate. The results from standard materials A, B, and the negative material meet the threshold of 5.5 in the applicable guidelines, indicating that the experiment parameters were valid.
Per the above, earthplusTM was found to exhibit cytotoxicity in V79 cells, with IC50 of 53.5% (95% confidence limit: 47.3-63.0%). Furthermore, cytoxicity of earthplusTM in V79 cells was higher than in standard material B, but lower than standard material A.
Acute toxicity tests
Acute toxicity test on rats using calibered dosage of earthplusTM
- Test number
- 9L384
- Test outsourcee
- Shinshu Ceramics Co., Ltd.
- Test date
- 08/06/99
- Test performer
- Mitsubishi Safety Science Laboratory, Hiroyuki Ishii
Test objective
Evaluation of acute toxicity in calibered dosage of earthplusTM in rats
Results and observations
- 1. Death
- During the observation period, no cases of death were observed.
- 2. General state
- During the observation period, no abnormalities were observed.
- 3. Weight (Tables 1 and 2)
- During the observation period, weight steadily increased.
- 4. Necropsy results
- No abnormalities were found.
- 5. Observations
-
After dosing 2000mg/Kg of earthplusTM to male and female rats in order to measure its acute toxicity, no cases of death were observed, nor were any abnormalities found in general conditions, weight change, or under necropsy.
The minimum lethal dose (LDLo value) for earthplusTM in this test was thus 2000mg/Kg or greater.
Acute toxicity tests
Test of revertant colonies in earthplusTM bacteria
- Test number
- B10756
- Test outsourcee
- Shinshu Ceramics Co., Ltd.
- Test performer
- Nippon Yuryo Kentei Kyokai
Results and observations
In this test, the number of revertant colonies induced through the specimen did not exceed two or more times greater than the spray control amount in both tests containing and not containing S9 mix.
The same results were obtained in dose-setting tests, confirming reproducibility of the results.
- No contamination was observed in the aseptic test.
- No growth impairment was observed in the test strains.
- Precipitation was observed on the plate, and this dose was indicated with a +.
The number of bacteria used was as follows.
| Strain name | Base-pair substitution | Frameshifting | ||||
|---|---|---|---|---|---|---|
| TA100 | TA1535 | WP2uvrA- | TA98 | TA1537 | ||
| Bacterial count | Dose-setting test | 3.7 | 3.7 | 5.5 | 3.9 | 2.0 |
| (x 109/ml) | This test | 3.8 | 4.3 | 4.7 | 3.9 | 2.6 |
In addition, the number of revertant colonies induced by the positive control in each strain with coexistence/non-coexistence of S9 mix clearly showed, compared to the number of revertant colonies in the spray control groups for each strain, a twofold increase, suggesting positive results. The mean of the spray control and positive control groups was within the range of past data found at this center, indicating that the test was conducted properly.
Based on these results, mutagenicity of earthplusTM was found to be negative.
Acute toxicity tests
Evaluation of skin irritation of earthplusTM in healthy subjects
- Test outsourcee
- Shinshu Ceramics Co., Ltd.
- Test date
- 12/04/02
- Test performer
- Yasushi Watanabe, Japan Hair Science Association
- 1. Test objective
- Evaluation of skin irritation in healthy subjects
- 2. Specimen
- earthplusTM treated fabric
- 3. Test modality
-
Subjects: 12 males and 13 females (total: 25) between the ages of 27 and 61
- Area of application: 10x10mm
- Patch test method: using Finchamber (manufactured by EPITEST Ltd.Oy) tape, a 10x10mm was square was used to occlude the upper arm flexor for 48 hours. The sample was removed after 48 hours had elapsed, and the skin condition observed and evaluated one hour after removal and 24 hours after removal.
- 4. Test results
- The results of patch tests on all 25 subjects were negative. The reaction 24 hours after removal of the sample (the table lists this as 72 hours of time) was negative in all cases, with no abnormalities.
Overall evaluation by test representative
The results of the past test we were contracted to perform for your company are as above, indicating that earthplusTM treated fabric has a low likelihood of causing an irritation.
I certify that I created this document per these test results.
Keiji Nagashima, MD
Primary skin irritation tests
Primary skin irritation using earthplusTM on rabbits
- Test number
- No.: 397050222-002
- Test outsourcee
- Shinshu Ceramics Co., Ltd.
- Test date
- 06/13/99
- Test performer
- Japan Food Research Laboratories, Tokyo headquarters
Summary
A primary skin irritation test was performed on earthplusTM printed antibacterial towels in compliance with the OECD test guidelines for chemicals (1981).
Three rabbits were administered occlusion tests for four hours on injured and intact parts of the sheared skin. No irritation of the rabbits’ skin was found after four hours had elapsed.
- 1. Test methodology
-
The hair coat of the trunk of each test animal was shaved 24 hours prior to the test.
After weighing the animals, each was designated with four sections six centimeters square, two of which were scratched on the keratinous layer so as to not reach the dermis (injured skin), and the other two sections left untreated (intact skin).
The sample was cut into approximately 2cm x 3cm, and the sheared surface moistened with approximately 0.5ml of purified water, with one injured and one intact skin section being sealed with bandages (Japanese Pharmacopoeia). 3M-Blenderm surgical tape was also used in order to hold the sample from coming in contact with the skin. The remaining injured and intact segments of skin were used for the test. Exposure time was set as four hours, after which time the samples were removed and the exposed skin was cleaned with purified water. Visual observation was performed 1, 24, and 48 hours after removal, with the degree of irritation being ranked per Table 1.
- 2. Test results
- No irritation was observed in any of the three test animals at each time interval after removing the sample.
Publications
- International Journal of Nanomedicine, 2011
- Paper presented in collaboration with Shinshu University School of Medicine concerning bacterial activity on cotton fabric treated with earthplusTM (titanium oxide, hydroxyapatite, and silver metal) and polypropylene nonwoven fabric
- The Japanese Association for Infectious Diseases, 2011
-
Evaluation of antibacterial performance of earthplusTM (e+) ceramic composite textile material
Poster presentation with Shinshu University Medical Department
- Household and Personal Care Products, 2012
- Antibacterial efficacy of TiO2/Ag treated fabrics (earthplusTM), paper presented with University of Arizona
- Japanese Society for Infection Prevention and Control, 2013
- Poster presentation: “Ongoing advances in the antiviral material earthplusTM -- basic findings in antiviral performance” (antiviral effects in cotton suits)
- Healthcare Infection Society Society, 2014 *1
- Details on antibacterial performance of earthplusTM against multidrug-resistant bacteria Poster presentations by Tohoku University Graduate School and Fukushima Medical University
*1 The Healthcare Infection Society is a British society for infectious control science. This body is considered one of the most prestigious groups involved with infection studies.
*2 Multidrug-resistant bacteria is a type of bacteria resistant to several antibacterial agents.
Click here for enquiries
81-264-55-1221
FAX. 81-264-55-1181
Enquiries by e-mail